Ba/F3 Xenograft Model
In Vivo Services
Ba/F3 Xenograft Overview
Protein kinases have become popular targets in the treatment of cancer and other diseases. Since 2001, the FDA has approved more than 70 kinase inhibitor drugs. However, due to innate or acquired resistance in tumors, most of these small molecule inhibitors only delay tumor progression. The development of next-generation kinase inhibitors with better specificity and lower resistance is ongoing.
Ba/F3 is a mouse pro-B cell line that relies on IL-3 for survival and proliferation. Following transduction with driver genes, such as kinase genes or their mutants, Ba/F3 cells shift from IL-3 dependence to dependence on the driver gene. This transformation makes Ba/F3 cells a potent tool for discovering new kinase inhibitors.
The KYINNO team has constructed over 700 Ba/F3 engineered cell lines that have been stably transfected with kinase gene mutants. These Ba/F3 kinase cell lines have undergone comprehensive validation through sequencing, western blotting, and inhibitor testing. They cover many popular kinases, including EGFR (>150 cell lines), RAS (80 cell lines), FGFR (55 cell lines), ERBB2 (49 cell lines), MET (41 cell lines), RET (39 cell lines), BCR-ABL (37 cell lines), EML4-ALK (33 cell lines), FLT3 (26 cell lines), and more. Most of these transformed Ba/F3 cell lines can be employed for xenograft models in immunodeficient mice.
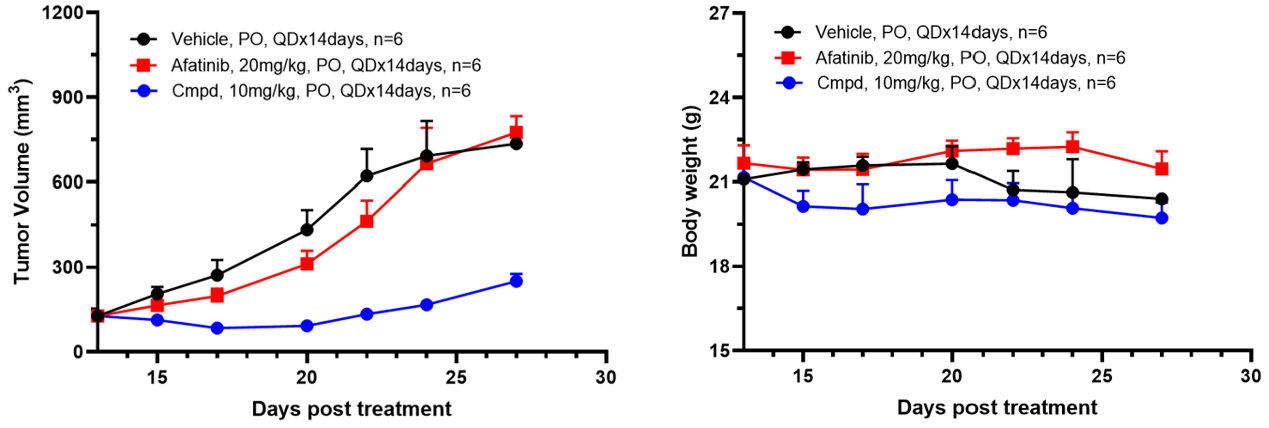
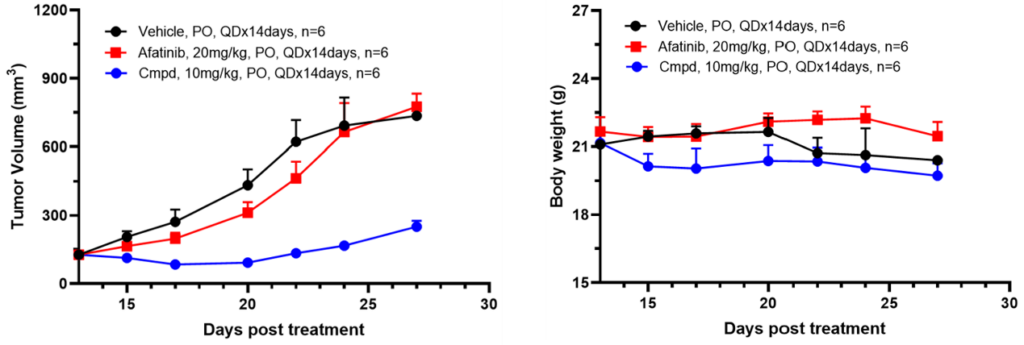
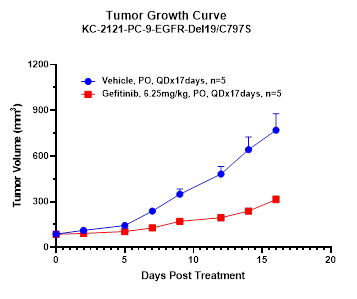
Utilizing these Ba/F3 kinase cell line-derived xenograft models, we have established an in vivo screening platform to assess the efficacy and toxicity of candidate drugs against specific kinase mutation types. Additionally, we compare these candidates with previous-generation drugs. In summary, our data indicate that xenograft models derived from Ba/F3 kinase cell lines are potent tools for discovering next-generation kinase inhibitors.
All in vivo Services
Ba/F3 Xenograft focuses on protein kinases, a cornerstone in cancer research. With FDA’s acknowledgment of kinase drugs, it offers important oncology insights aiding for deeper understanding.
Gene-edited xenografts, utilizing tools like CRISPR/Cas9, offer precise modifications to tumor cell genomes, significantly expanding the horizons and potential of modern oncological research methodologies and insights.
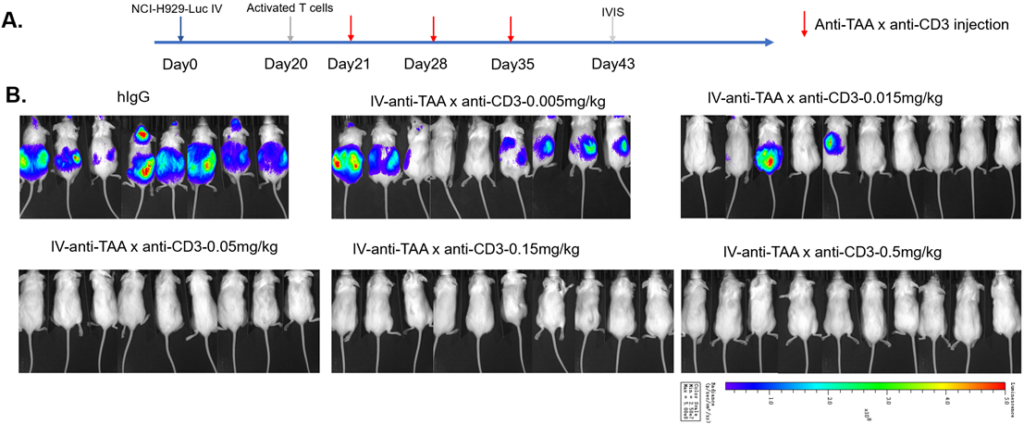
Luciferase tumor model provides insights into tumor growth and immune dynamics. Using bioluminescence, it provides real-time insights. This innovative approach deepens comprehension, advancing cancer research and promoting novel therapeutic discoveries.
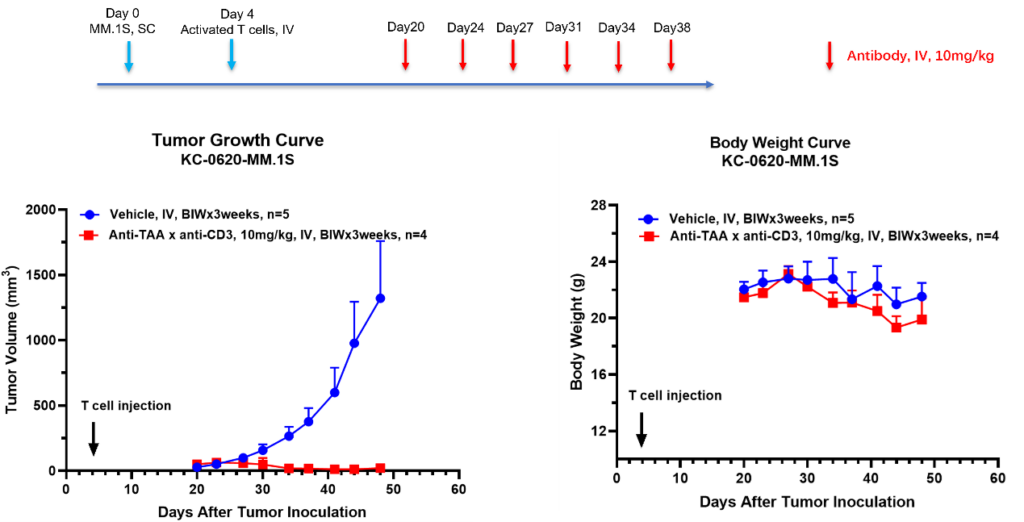
PBMC Humanized Mouse Model incorporates human T cells’ behavior, enabling detailed tumor immunology analysis. It presents a unique avenue for in-depth exploration, enriching research and offering a comprehensive study platform.
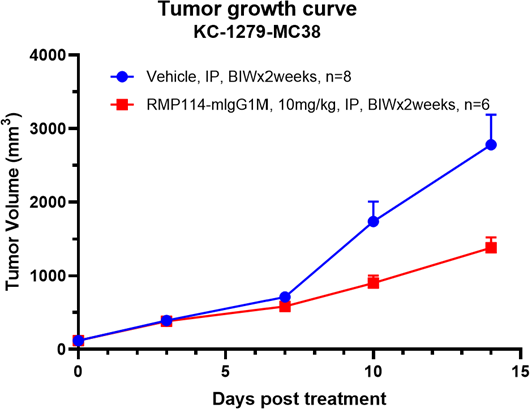
The Syngeneic Model leverages the uses of mouse-derived tumor tissues, placed into genetically similar hosts, contributing to consistency and depth in cancer studies. Ensures a genuine environment for cancer study.
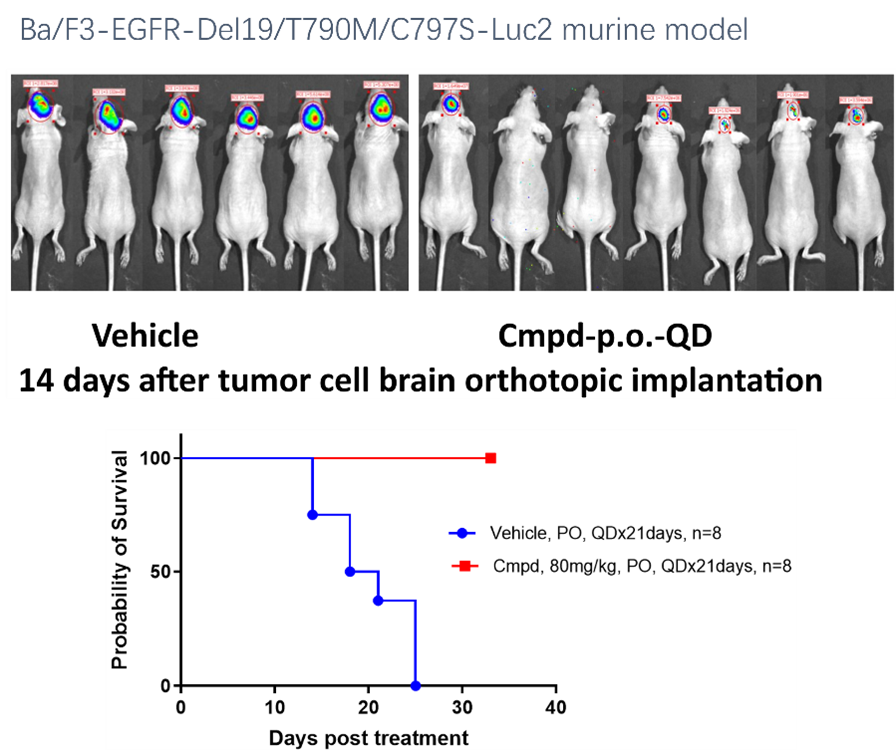
The Subcutaneous tumor models are a leading choice for evaluating anti-cancer drug efficacy, supporting clinical trials, and understanding potential treatments. Its established method is a key component in therapeutic research.
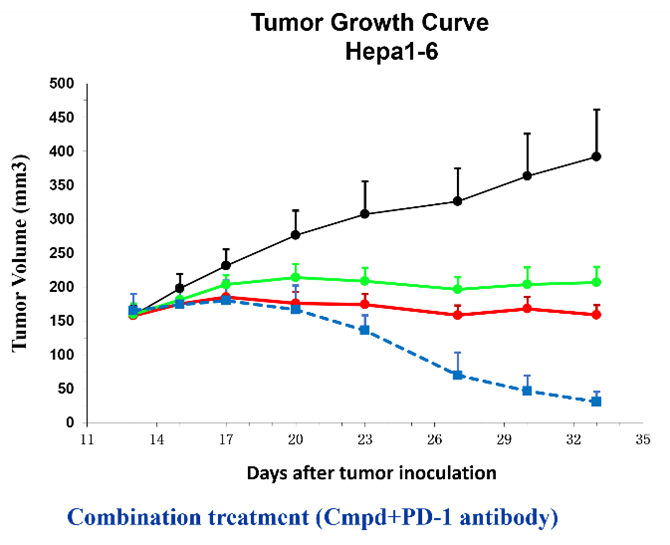
The subcutaneous tumor model is one of the most commonly used in vivo evaluation systems for assessing the efficacy of novel anti-cancer drugs. Typically, tumor …
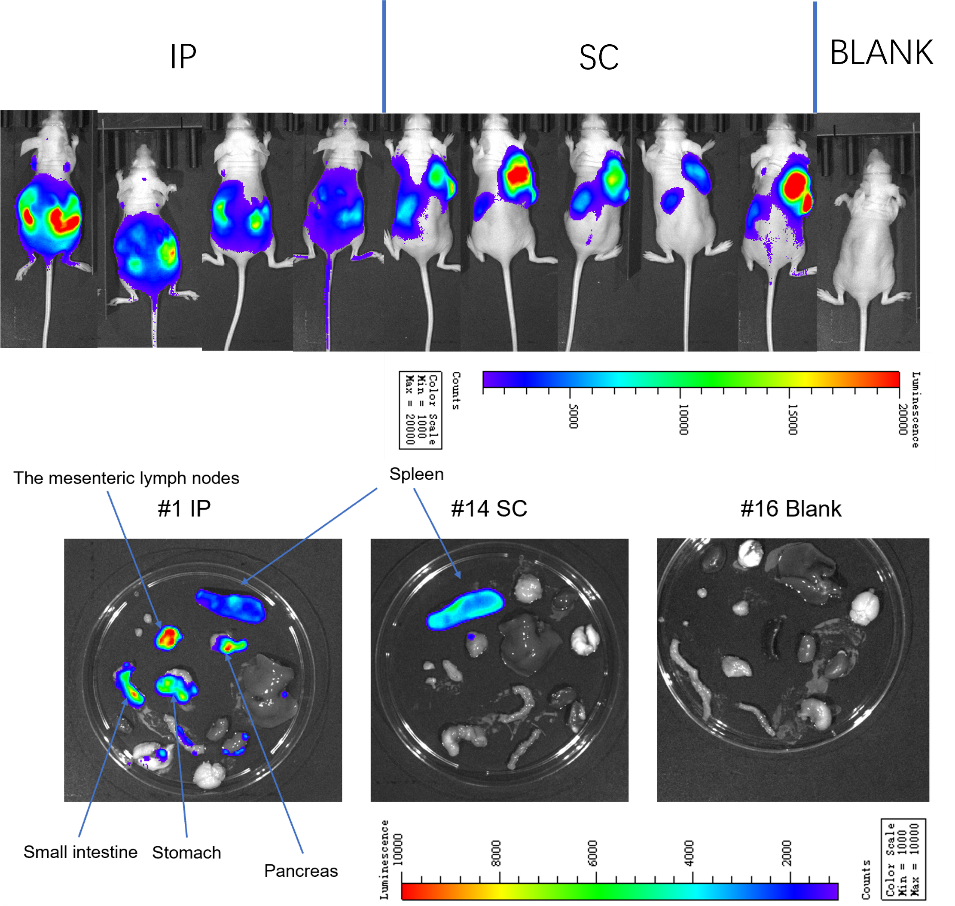
In cancer research, metastasis models are crucial tools that help us understand how tumors migrate from their original location to other parts of the body. Ba/F3 cells are a common type of tumor cell, especially in mouse models, where they exhibit metastasis.